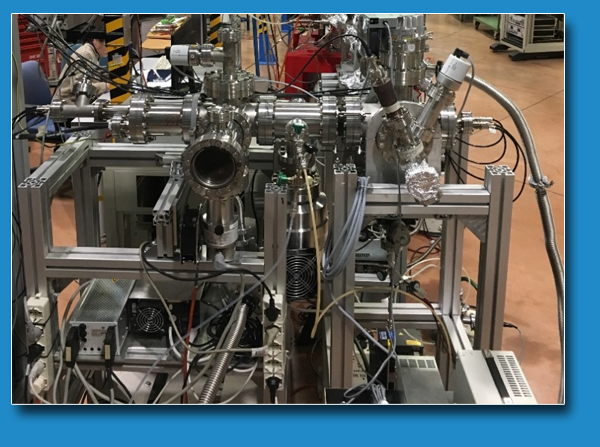
The molecular systems are admitted in the chamber in vapor phase through a heated needle in the case of liquid samples or using a heated furnace in the case of powders.
The ionization region is equipped with ion optics (one planar repeller and three extractor lenses), set in front of the octupolar RF-driven ion guide. The effusive beam is in the center of the ionization region crossed by the photon beam. The ions produced are transported into the octupole by the extraction optics, set perpendicularly to the propagation axis of the photon beam. The octupolar ion guide transfer the ions into the quadrupolar mass spectrometer where they are collected. In the case of ion-molecule reaction experiments, the neutral reagents are introduced in the octupole that acts as a two-dimensional trap, which ensures an extended reaction region and an efficient collection of the ionic products of the ion–molecule reactions. All the experiments can be performed as a function of the photon energy scanning the monochromator together with the current of the insertion device of the CiPo beamline.
TECHNICAL SPECIFICATIONS
- Energy range: 8-17 eV (NIM-Al), 15-40 eV (NIM-Au)
- Mass range: 10-4000 m/z
- Sample temperature: from RT to 150 °C
- Sample pressure: up to 10-4 mbar
AVAILABLE TECHNIQUES
- Mass spectrum at fixed photon energy
- Photoionization efficiency curves as a function of m/z and photon energy
- Ion-Molecule reactions as a function of collision and photon energy.
SAMPLE
-
Liquid sample.
The sample is placed in a glass tube and connected to the ionization chamber through a needle. The gas line of the sample can be heated up to 150 °C. -
Powder sample.
The sample is evaporated by heating a furnace. The furnace can be heated up to 150 °C. -
Time for loading the sample and start measure: from 1h to 1 day.
USE FOR
-
Molecules
-
Complexes
-
Reactivity
-
Atmosphere
-
Biophysics
-
Case Studies
Case Studies
HSO Formation2+ Reactions of SO2·+ with Water and Methane: Two Fast Reactions with Reverse Temperature-Dependent Kinetic Trend
In this work an experimental and theoretical study on the formation of HSO2+ from the SO2·++ CH4 and SO2·++ + H2Oa ion-molecule reactions at different temperatures is reported. Calculations in the frame of the density functional theory and variational transition state theory were combined to explore the dynamics of the reactions. The dynamic behavior observed in these two reactions is of general and fundamental interest. It can also provide some insights on the role of these reactions in astrochemistry as well as in their use as models for bond-activation reactions.
See: Chem. Eur. J., 10.1002/chem.201700028
Nitromidazoles are relevant compounds of multidisciplinary interest, and knowledge of their physical−chemical parameters as well as their decomposition under photon irradiation is needed. Here we report an experimental and theoretical study of the mechanisms of VUV photofragmentation of 2- and 4(5)-nitromidazoles, compounds used as radiosensitizers in conjunction with radiotherapy as well as high-energy density materials. The results show that these compounds can be an efficient source of relevant CO, HCN, NO, and NO2 molecules and produce ions of particular astrophysical interes.
See: J. Phys. Chem. A, 10.1021/acs.jpca.8b01144

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)