
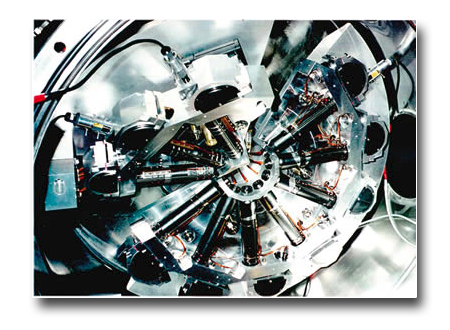
The Gas Phase beamline at Elettra is specifically devoted to research on gaseous systems. It is operated by a joint Research Team of Elettra Sincrotrone Trieste and Italian National Research Council (CNR-IOM and CNR-ISM)-
The active in-house and users' research program at GasPhase covers the following areas of activity:
- high resolution studies of electronic structure and dynamics of atoms and molecules (with core level photoabsorption, photoemission and dispersed fluorescence);
- characterization of reactive gases, radicals, transient species (electron and ion coincidence spectroscopies);
- studies of complex molecules (biological and other organic molecules, and organometallic complexes);
- studies of inorganic and organic clusters;
- studies of electron correlation in atoms and molecules after double photoionization, and inner shell ionization and decay;
- novel instrumentation for investigation of many body interactions in complex systems
The beamline is ideally suited forcarrying out detailed synchrotron radiation investigations on the spectroscopy and the dynamics inatoms, molecules and clusters isolated in the gasphase.
TECHNICAL SPECIFICATIONS
GasPhase
- GasPhase main line: energy range 13-900 eV
- GasPhase branchline: energy range 13-250 eV
- High flux (up to 1014ph/s @100 eV, 0.1% bandwidth,
- high resolving power: E/ΔE ≥ 10000
- small spot size at target: ≈250x250 µm.
The beamline is presently equipped with several distinct interchangeable set-ups:
1. Multi-coincidence experiments set up, for angle resolved and electron-electron coincidence experiments in a versatile multichannel configuration (fig. 2)
2. Velocity Map Imaging (VMI) set up (fig. 3)
3. VG-220i set up for photoemission and mass spectrometry of condensable vapours, equipped for ion-electron coincidences, PEPICo (Fig. 4).
4. Molecular beam set up, for PEPICo mass spectroscopy.
5. ARPES, angle-resolved photoemission experiments on aggressive molecular species (radicals, transients, reactive species)
6. SCIENTA SES-200 electron analyzer (Fig. 5; in collaboration with Uppsala University).
7. PIFS and XUV-PIK, for dispersed emission experiments with an UV-Vis monochromator and/or a compact XUV spectrometer (in collaboration with IFN-CNR, Padova).
8. CESyRa "Cluster Experiment with Synchrotron Radiation" for photoionization of clusters of refractory materials, via electron-ion multicoincidence (in collaboration with prof. Paolo Piseri, University of Milan and NFFA).
At variance with other beam lines at the ELETTRA, the end-station is not fixed, but several interchangeable apparatuses are available for users’ experiments and users can also bring their own apparatus to perform experiments, provided that it is compatible with beamline requirements.
On the branch-line, a ps-laser, tunable in the range 700-1000 nm (Tsunami, SpectraPhysics, ~83 MHz) is available. It can be synchronized to synchrotron radiation for pump-probe experiments.
More details at :
www.elettra.eu/elettra-beamlines/gasphase.html
AVAILABLE TECHNIQUES
- XAS: X-ray and VUV photo absorption
- UPS; XPS and Augers pectroscopy
- Mass spectrometry
- Fluorescence and photon in-photon out spectroscopy
- Photoelectron-photoelectron coincidence spectroscopy
- Photoelectron-photoion coincidence spectroscopy
- Velocity Map Imaging

VMI set up

VG 220i electron analyzer

SCIENTA SES-200 electron analyzer
SAMPLE
-
All systems in gas phase or that can be brought into the gas phase ( atoms, metals, molecules, biomolecules, inorganic and organic clusters, radicals and reactive species)
-
Gas inlet for gases or vapor from liquids or high vapor tension solids
-
Diffusive beam of molecules from an anti-inductive oven (T=20°-480°C)
-
Atomic and molecular clusters produced by different supersonic jet techniques.
USE FOR
-
studies of electron correlation in atoms and molecules in double photoionization and inner shell ionization and decay
-
high resolution studies of electronic structure and dynamics of atoms and molecules (with core level photo absorption, photoemission and dispersed fluorescence);
-
characterization of reactive gases, radicals, transient species (electron and ion coincidence spectroscopies);
-
studies of complex molecules (biological and other organic molecules, and organometallic complexes)
-
studies of inorganic and organic clusters
Case studies
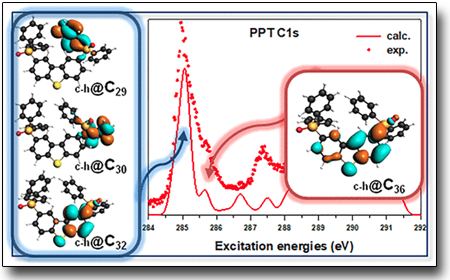
Comprehensive gas phase electronic structure characterization of PPT (2,8-bis(diphenylphosphoryl) -dibenzo[b,d]thiophene) a promising ambipolar phosphorescent host material recently introduced in organic light-emitting diodes (OLEDs). This system can be considered formed by two diphenylphosphine oxide (dPPO) moieties functionalizing the small dibenzothiophene (DBT) core. PPT is characterized by high triplet energy and is known as good vacuum sublimable electron transporting material for blue OLEDs. The triphenyl phosphine oxide (TPPO) molecule has been chosen as the model compound of the dPPO groups in PPT. A combined experimental and theoretical study by density functional theory of the gas phase electronic structure of TPPO and PPT has been performed through X-ray photoelectron spectroscopy and near-edge X-ray absorption fine structure spectroscopy measured at the carbon and oxygen 1s regions. The study represents a detailed characterization of the impact of the single building blocks on the electronic structure of the whole PPT molecule. Moreover, it confirms that the phosphine oxide groups actas breaking points of the π-conjugation between the DBT core of PPT and the outer groups, leaving the electronic structures of the compound practically matching those of the central DBT moiety
See: "PPT Isolated Molecule and Its Building Block Moieties Studied by C 1s and O 1s Gas Phase X‑ray Photoelectron and Photoabsorption Spectroscopies"
A. Guarnaccio et al. J. Phys. Chem. C 124 (2020) 9774
https://pubs.acs.org/doi/10.1021/acs.jpcc.0c01764
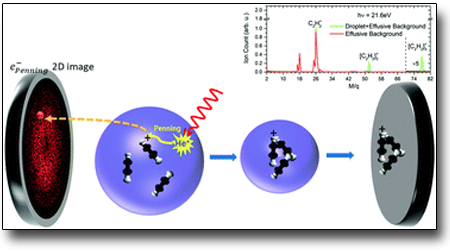
Embedded atoms or molecules in a photoexcited He nanodroplet are well-known to be ionized through inter-atomic relaxation in a Penning process. In this work, we investigate the Penning ionization of acetylene oligomers occurring from the photoexcitation bands of He nanodroplets.
In close analogy to conventional Penning electron spectroscopy by thermal atomic collisions, the n = 2 photoexcitation band plays the role of the metastable atomic 1s2s 3,1S He*. This facilitates electron spectroscopy of acetylene aggregates in the sub-Kelvin He environment, providing the following insight into their structure: the molecules in the dopant cluster are loosely bound van der Waals complexes rather than forming covalent compounds. In addition, this work reveals a Penning process stemming from the n = 4 band where charge-transfer from autoionized He in the droplets is known to be the dominant relaxation channel. This allows for excited states of the remnant dopant oligomer Penning-ions to be studied. Hence, we demonstrate Penning ionization electron spectroscopy of doped droplets as an effective technique for investigating dopant oligomers which are easily formed by attachment to the host cluster.
Si veda:
"Penning spectroscopy and structure of acetylene oligomers in He nanodroplets"
S. Mandal et al. PCCP. 22,(2020) 10149
https://doi.org/10.1039/D0CP00689K
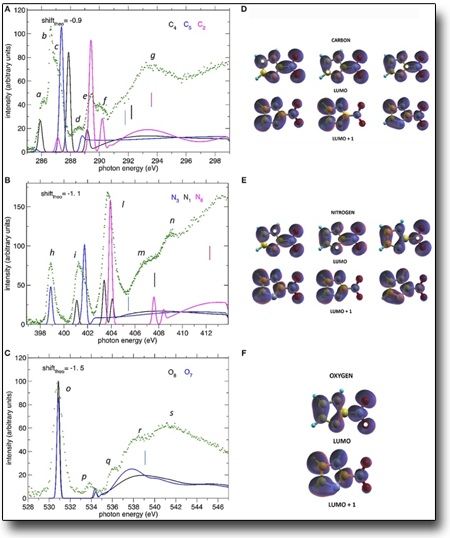
Tunability and selectivity of synchrotron radiation have been used to study the excitation and ionization of 2-nitroimidazole at the C, N e O. The combination of a set of different measurements (X-ray photoelectron spectroscopy, near-edge photoabsorption spectroscopy, Resonant Auger electron spectroscopy, and mass spectrometry) and computational modeling have successfully disclosed local effects due to the chemical environment on both excitation/ionization and fragmentation of the molecule. "Core Shell Investigation of 2-nitroimidazole"
See: "Core Shell Investigation of 2-nitroimidazole"
P. Bolognesi, et al. Frontiers in Chemistry, 7 (2019) 151
https://www.frontiersin.org/articles/10.3389/fchem.2019.00151/full

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)