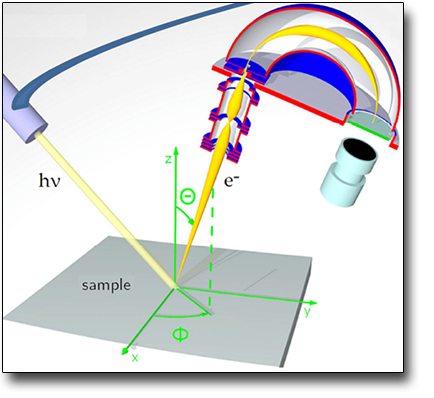
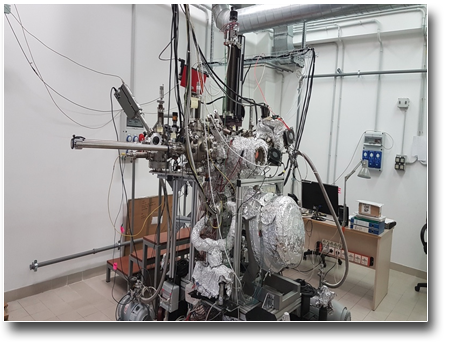
TECHNICAL SPECIFICATIONS
- Al-Mg X-ray source
- electron gun
- five-channeltrons VG electron analyzer
- preparation chamber
- preparation chamber and analysis chamber share the same manipulator
- fast entry lock and a supplementary chamber for samples treatments
AVAILABLE TECHNIQUES
-
XPS
-
LEED
-
EELS
SAMPLE
-
Solid samples: lateral dimensions 9 x 9 mm (ideal), 6 x 6 mm (minimal), 11 x 11 mm (maximal)
-
Sample thickness: ideally up to 2 mm (thicker samples also feasible)
-
Sufficient electrical conductivity of samples needed to avoid charging
USE FOR
- Study of the surface of metals and semiconductors
- Ultra-thin Films
- Chemical reactions on surfaces
- Catalysis
Case Studies
Two-dimensional π-conjugated polymers
Two-dimensional π-conjugated polymers constitute a promising subclass because the band structure can be manipulated by varying the molecular building blocks while preserving key features. The fabrication of mesoscale ordered two-dimensional π-conjugated polymer kagome lattices with semiconducting properties, Dirac cone structures and flat bands on Au(111) has been demostrated. These results open opportunities for the synthesis of two-dimensional π-conjugated polymer Dirac cone materials and their integration into devices.
See: G. Galeotti, ... e G. Contini, Nature Materials 2020, https://doi.org/10.1038/s41563-020-0682-z
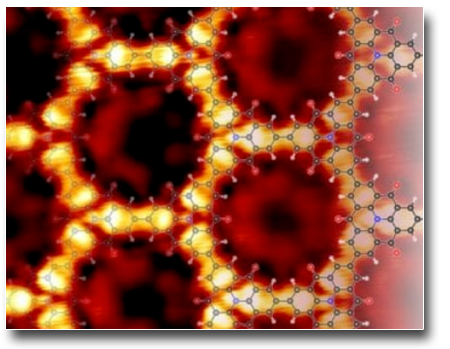
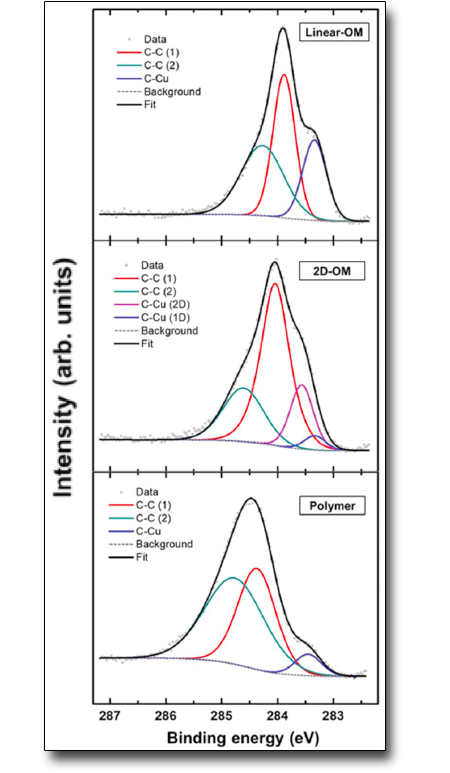
Organometallic intermediate in surface-confined Ullmann coupling
Ullmann coupling is generally described as a two-step reaction: (i) dehalogenation, resulting in the formation of a stable intermediate organometallic phase and subsequent (ii) C–C coupling. The topology of the resulting polymer is determined by the structure of the intermediate phase. Hitherto, only one intermediate structure, identified as an organometallic (OM) phase, has been reported for such a reaction. Here we demonstrate the formation of two distinct OM phases during the temperature-induced growth of poly(para-phenylene) from 1,4-dibromobenzene precursors on Cu(110). This new intermediate phase, revealed only when the reaction is carried out at low molecular coverages, was characterized by X-ray photoelectron spectroscopy, scanning tunneling microscopy and near-edge X-ray absorption fine structure spectroscopy, and modeled by density functional theory calculations.
See: G. Galeotti, ... e G. Contini, Nanoscale, 2019, 11, 7682

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)