Molecular self-assembly and molecular recognition are two concepts in supramolecular chemistry in which molecules couple through non-covalent interactions. On surfaces, hydrogen bonding, metal coordination, halogen bonding and dipole-dipole interactions hold particular relevance in the formation of highly-organised 2D networks. Chalcogen bonding interactions—already used effectively in crystal engineering—have not yet been demonstrated to drive molecular self-assembly on surfaces, however.
In a joint experimental-theoretical collaboration, involving surface physicists from CNR-ISM and the University of Rome “Tor Vergata” and organic chemists from the University of Vienna, molecular recognition driven solely by chalcogen bonding has been demonstrated for the first time in a new communication published in JACS Au. High-resolution and “bond resolved” scanning tunnelling microscopy measurements reveal that a chalcogenazolo pyridene derivative undergo chiral dimerization when deposited on the Au(111) surface. Density functional theory calculations, including analyses of the non-covalent-interactions index and topological analysis of the electron density, prove that the dimers bind through noncovalent double Te-N or Se-N interactions.
These findings will pave the way for designing and fabricating precise supramolecular nanostructures on surfaces with tailored semiconducting properties, and opens a new avenue in bottom-up engineering of 2D monolayered supramolecular materials based on chalcogen bonding.
Wednesday, 12 June 2024 14:37
On-Surface Molecular Recognition Driven by Chalcogen Bonding - A new paper
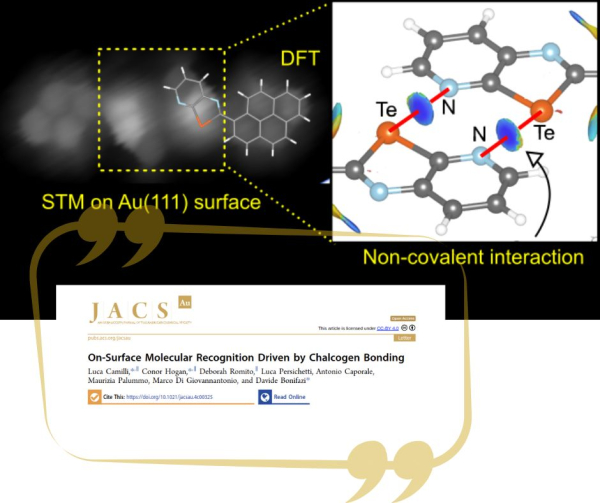 DFT calculations reveal that molecular dimers observed to form on a gold surface are bound by non-covalent chalcogen bonding interactions.
DFT calculations reveal that molecular dimers observed to form on a gold surface are bound by non-covalent chalcogen bonding interactions.
Chalcogen bonding interactions (ChBIs) have been widely employed to create ordered noncovalent assemblies in solids and liquids. Yet, their ability to engineer molecular self-assembly on surfaces has not been demonstrated. Here, we report the first demonstration of on-surface molecular recognition solely governed by ChBIs. Scanning tunneling microscopy and ab initio calculations reveal that a pyrenyl derivative can undergo noncovalent chiral dimerization on the Au(111) surface through double Ch...N interactions involving Te- or Se-containing chalcogenazolo pyridine motifs.
Published in
Publications

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)