Nanochemistry for the synthesis of nanoparticles and nanocomposites
Aldo Capobianchi -
Laboratory BC12 (nM2-Lab)
TECHNICAL SPECIFICATIONS
- Stirring/heating plates: various models, Tmax 300°C.
- Lines with rotary vacuum (P = 2x10-3mmHg).
- LB deposition apparatus: (Nordtest, KSV 5000)
AVAILABLE TECHNIQUES
-
Chemical synthesis and deposition of organic thin films and nanoparticles using the Lagmuir-Blodgett (LB) technique.
SAMPLES
-
Powders or crystals in typical quantities of the order of 100 mg. The easy scalability of the methods allows the preparation of larger quantities.
-
Organic thin films and nanoparticles with a maximum surface area of 10x10 cm2 (by LB).
USED FOR
- Permanent magnets
- Catalysis
- Sensors
- Semiconductor / microelectronics
- Cleaning and purification of water
- Chemical industry
CASE STUDIES
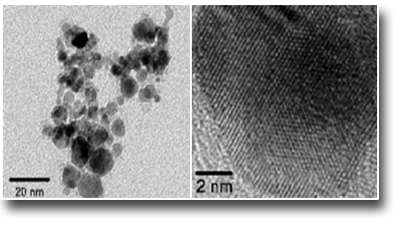
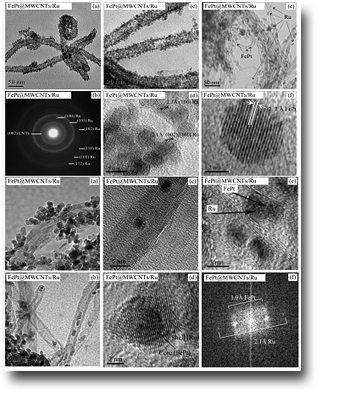
Nanoarchitectures of FePt@MWCNTs/Ru with double functionalization.
The example shows the synthesis of nanocomposites with a complex three-component nanoarchitecture: carbon nanotubes (CNTs) that give a large surface, Ru nanoparticles (NPs) that decorate the CNTs and act as a catalyst and FePt NPs inside the CNTs that have the purpose of giving the nanocomposite a magnetic behavior. This last one has a dual function: the first and simpler is to move or remove the catalyst nanocomposite how it prefers in the reaction environment. The second and more complex is to provide local heating to the catalyst without heating the whole solution. Local heating is obtainable through an alternating magnetic field applied from the outside as occurs in the case of hyperthermia of magnetic NPs for therapeutic purposes. In the catalysis phase this can lead to a strong saving of energy and to a greater specificity of the reaction. The uniqueness of this work lies in the great control over the structure of the nanocomposites and the highly specific positioning (internal or external to the CNTs) of its components.
See: B. Astinchap,R. Moradian, A. Ardu, C. Cannas, G. Varvaro, A. Capobianchi. Chem. Mater. 24, 3393(2012)
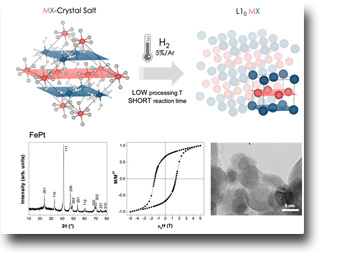
Effective synthesis of L10 alloy nanoparticles from stratified precursor salts.
An intelligent and easily scalable synthesis strategy, called Preordered-Precursors Reduction, has been successfully applied to synthesize highly ordered L10 MPt (M = Fe, Co Ni, Mn) alloy nanoparticles under milder conditions than ordinary processes. The natural order of the crystalline M(H2O)6PtCl6 precursor salts, consisting of M and Pt atoms on alternating planes that imitate the atomic disposition of the L10 structure, plays a fundamental role in providing all systems with a certain initial quantity of chemical order that facilitates the formation of the ordered phase L10, which is therefore obtained in milder conditions, in terms of process temperature and reaction times, compared to what is required by ordinary strategies.
See:
- X.C. Hu, E. Agostinelli, C. Ni, G.C. Hadjipanayis, A. Capobianchi. Green Chem. 16, 2292 (2014)
- G. Varvaro, P. Imperatori, S. Laureti, C. Cannas, A. Ardu, P. Plescia, A. Capobianchi, JALCOM, In press (2020)

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)