In a TOF-mass spectrometer a radiation source is used to ionize the sample. Depending on the energetics in the process, the parent molecule will undergo molecular fragmentation, releasing one or more charged and neutral species:
hv + M --> m1+ + m2 + e-
TECHNICAL SPECIFICATIONS
-
The TOF-mass spectrometer is equipped with:
- bakeable high vacuum chamber in camera pari a 10-9 mbar);
- custom Wiley-McLaren time-of-flight (TOF) analyser (mass resolution of 1 amu up to about m/z 100);
- electron channel multiplier (CEM) e microchannel plates (MCP);
- stainless steel oven heated by a Thermocoax (up to 300°C);
the oven can house a crucible to sublimate solid samples, or a gas line to transfer liquid/gaseous samples of sufficient vapor pressure from test tube located outside the vacuum chamber;- VUV rare gas discharge lamp as photon source
Gas Pressure (mbar) Energy (eV) Current (pA) He 3x10-1 21.22 5 Ne 3x10-2 16.67 3 Ar 1x10-2 11.62 0.2 Kr 1x10-2 10.00 0.2 Xe 2.5x10-3 8.43 0.05
- VUV rare gas discharge lamp as photon source
- electronics and signal processing (NIM standard)
- time-to-digital converter with multi-hit capabilities (ACAM)
- computer equipped with LabView and C++ for data acquisition and data analysis
- heating system to bake-out the apparatus
- the pulsed extraction can be implemented if needed
AVAILABLE TECHNIQUES
-
Gas-phase mass spectrometry in the VUV range.
- Mass spectrometry is a powerful analytical tool to characterize compounds and to determine their structure via the detection and analysis of their fragments, to determine mixture composition, to achieve qualitative identification of molecules and to study radiation-induced fragmentation patterns.
It can be used for gas phase samples as well solid/liquid samples that can be brought to gas phase by thermal evaporation without decomposing.
SAMPLE
-
Small molecules in gas and solid/liquid phase that can be evaporated/sublimated without decomposing.
-
Max sublimation temperature 300°C.
USE FOR
-
Characterization of mass spectra of molecular species (m/z< 300).
Case Studies
VUV photofragmentation of halogenated methanes and the atmospheric chemistry
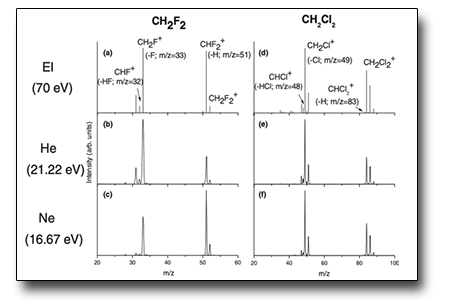
Halomethanes are widely recognized anthropic species long living in the atmosphere, with potentially harmful impact on the ozone layer. The study of the photofragmentation of CH2X2 molecules (X=F, Cl, Br, I) and chlorinated methanes (CHnCl4−n with n = 0–3) using He, Ne, and Ar VUV discharge lamps can shine light on their chemistry in the upper atmosphere. The results have shown the peculiar behavior of the CH2F2 molecule which is the only dihalomethane to dissociate via the H-loss channel yielding CHF2+ ion and an H atom, with relevant atmospheric implications. Indeed, as fluorine reacts rapidly with atmospheric molecule to form HF, the reactive H atom can also affect the atmospheric environment via different chemical reactions with important molecules like ozone.
See: J. Chem. Phys. 140, 184307 (2014)
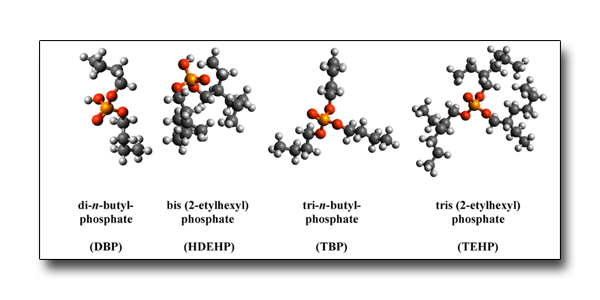
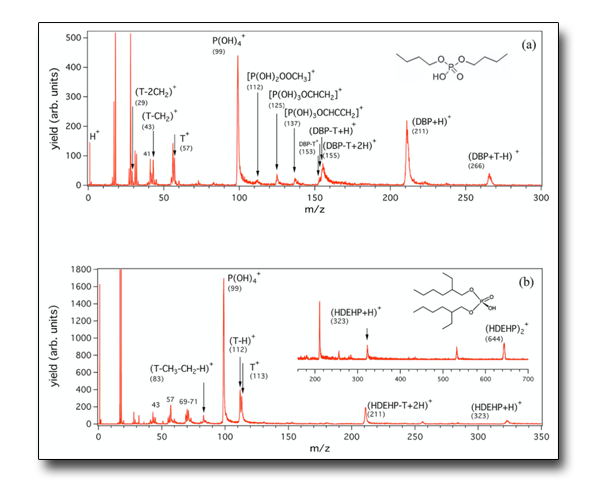
Alkyl phosphates are used in chemical industry. Once released in the environment, they can become source of pollution, and several approaches have been proposed for their degradation, including UV/VUV light irradiation.
This work investigates the fragmentation mechanisms of alkyl phosphates in the VUV range. The results show that all the investigated substances have similar fragmentation patterns characterized by the stepwise cleavage of the C-O bond, which links the alkyl chains to the phosphate group, and lead to the formation of esters with a lower number of alkyl side chains and ending to the protonated orthophosphoric acid.
See: Journal of Photochemistry & Photobiology A: Chemistry 365 (2018) 13–22
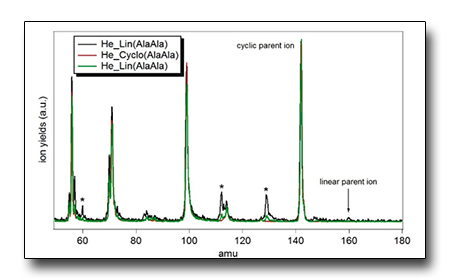
A gas-phase study of peptide bond formation/disruption.
Since these processes rely on the mechanisms of peptide bond formation and cleavage, we have undertaken a study of the peptide bond formation/disruption on the simplest peptides, the cyclic (c-) and linear (ℓ-) dipeptides in the gas phase, by photoionisation experiments.
The preliminary results suggest that a cyclisation process may happen during the TOF-MS measurements carried out on linear dipeptides. This process may be driven by the temperature in the condensed phase, the ‘electrostatic forces’ in the unstable zwitterion produced in the sublimation or the fast rearrangement of the highly reactive cation following ionisation.
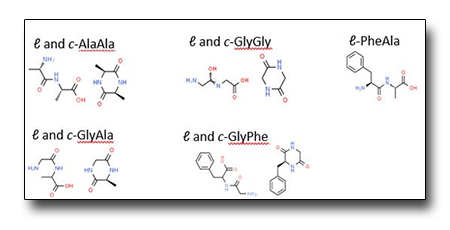
Schematic of the ℓ- and c-dipeptides studied.

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)