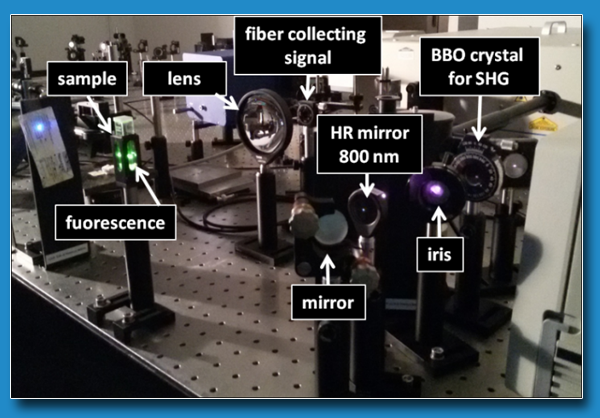
TECHNICAL SPECIFICATIONS
- Available laser sources:
- Spectra Physics, Spitfire Pro, Ti:Sa Laser: ʎ 800 nm (tunabile con sistema OPA nel range 290-2500 nm); 120 fs; 4 mJ; repetition rate up to 1 KHz;
- Tsunami: ʎ 800 nm (400 nm by SHG using a BBO crystal); 90 fs, 10 nJ, 80 MHz;
- Electronic for timing: PicoQuant Pico Harp 300;
- Fast photodiode: TDA 200;
- Monochromator: Princeton Instruments ACTON SP2150;
- Photo-multiplier tube: PicoQuant PMA-C 192-N-M (< (< 180 ps (FWHM))
AVAILABLE TECHNIQUES
- Fluorescence decay (Int. vs Time);
- Fluorescence anisotropy and in polarization control;
- Fluorescence 3D maps (time vs ʎem vs Int.) of fluorescence decays over a wide wavelength range of excitation (ʎex 290-2500 nm) and emission (ʎem 230-920 nm).
SAMPLE
-
Preferred: diluted solutions(10-4-10-6 M) in organic solvents;
-
Thin films
USE FOR
-
Organic/Inorganic Semiconductors;
-
Thin films/coatings;
-
Nanoparticles.
Case Studies
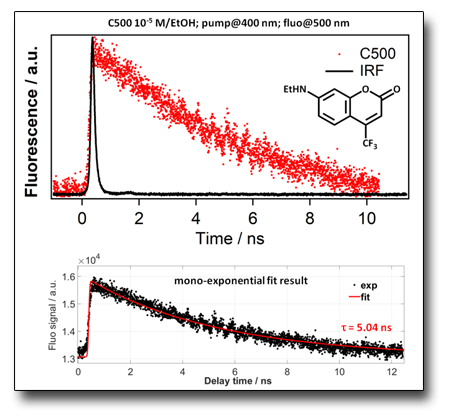
COUMARIN 500 (C500) fluorescence decay
The study case of C500 has been used in order to evaluate the performance of our experimental setup using a Tsunami (80MHz, ʎex 400 nm BBO SHG). The fit analysis has highlighted that a mono-exponential decay model is useful to describe the radiative fluorescence decay (τ=5.04 ns)registered by exciting a 10-5 M of C500 in EtOH, solution by a 400 nm ultra fast laser source as reported in literature for the same dye system under analysis.
See: Sanjucta, Nad et al., J. Phys. Chem. A, 107, 501 (2003)
DOI: 10.1021/jp021141l
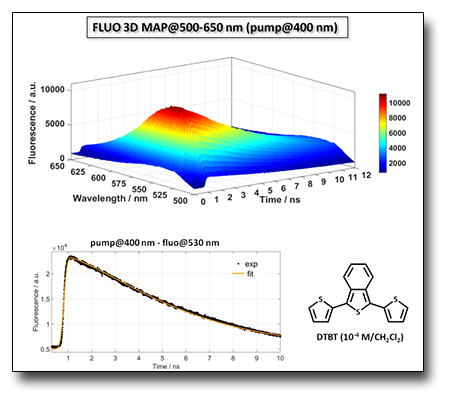
FLUORESCENCE 3D MAP of a short oligothiophene (DTBT) molecule
The experimental setup described for the C500 study case has been used to investigate the fluorescence lifetime related to a solution (10-4 M/CH2Cl2 of a short chain oligothiophene compound useful for organic photovoltaic applications (i.e. the DTBT, 1,3-di(2-thienyl)-2-benzothiophene). The evaluation of the fluorescence lifetime has been the starting point for a much wider comprehension of radiative and non-radiative processes occurring within the excited DTBT systems (by fs laser excitation source) in solution. Indeed, the fluorescence "competes" with non-radiative decay processes studied by FTAS (Femtosecond Transient Absorption Spectroscopy) measurements.

 English (UK)
English (UK)  Italiano (Italia)
Italiano (Italia)