Photoinduced electron transfer and charge transfer have a great importance in nature since they govern photosynthesis in plants and bacteria and are the driving mechanisms of basic processes at the boundary between quantum effects and molecular dynamics. The combination of synchrotron spectroscopies, ultrafast XUV pump- IR probe experiments and many-body quantum chemistry calculations has allowed to study in details the first steps of charge-transfer processes initiated by prompt ionization in nitroanilines,a prototype donor–π–acceptor molecules.
The theoretical and experimental results published in Nature Chemistry showed that in the prototype molecule of nitroaniline the charge transfer from the lone-pair orbital of the NH2 group to the C-N bonding occurs in 10fs and is driven by the planarization of the same NH2 group. The dynamics of the process is driven by the coupled electronic-nuclear motion generated by an ultra-short pulse lasting a few attoseconds in the molecular cation, in which the electronic charges are redistributed according to the nuclear rearrangements.
In addition to contributing to a better understanding of the qualitative concepts used to predict charge migration in organic molecules, this work offers a perspective for engineering the chemical and structural properties of these molecules to control and optimize the charge transfer.
The implications span from photosynthesis in plants and bacteria, to the response of photovoltaic devices and to electronic transport in single-molecule devices.
Wednesday, 23 October 2024 10:10
Energy and charge transfer observed in real time - A new paper
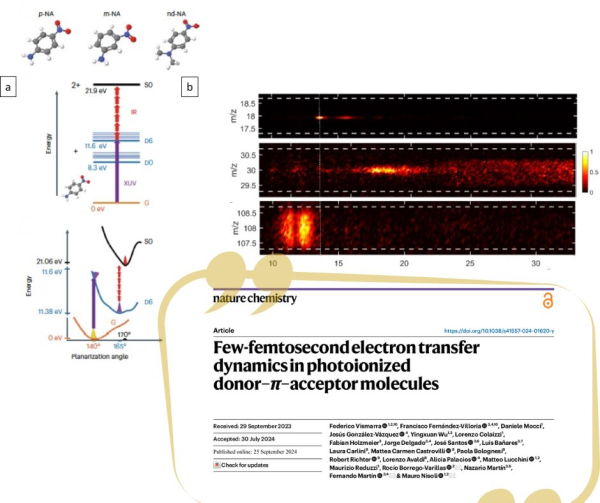 a) Scheme of the XUV pump-IR probe experiment; b) PEPICO measurements around m/q=18 (H2O+) , m/q=30 (NO+) and m/q=108 ((M-NO)+)
a) Scheme of the XUV pump-IR probe experiment; b) PEPICO measurements around m/q=18 (H2O+) , m/q=30 (NO+) and m/q=108 ((M-NO)+)
An international collaboration that involved researchers from eight different institutions (Department of Physics Polytechnic of Milan, CNR-IFN, CNR-ISM, IMDEA Nanociencia, Departamento de Quimica UAM, Departamento de Quimica Organica Universidad Complutense de Madrid, imec Louvain and Sincrotrone Trieste ) reported for the first time the measurement of the time necessary to transfer the charge from an electron donor system to the adjacent carbon atom in a benzene ring and therefore the time in which the structural change of the molecule occurs.
The results are published in Nature Chemistry.
Published in
Publications